At the Institut Jules Bordet, we are exploring the immune ecosystem of human lung cancers, with an emphasis on innate immune cells, particularly neutrophils. We hope to identify new biomarkers associated with functional perturbations of these cells in tumors. The function of new candidate proteins that may impact neutrophils and other tumor-associated immune cells will then be tested in preclinical model systems in the Gosselies campus.


At the Gosselies campus of ULB, we are using preclinical lung cancer models to test our new hypotheses from human tumor research performed at the Institut Bordet. Because our recent studies have positioned tumor-associated neutrophils as a critical component for lung tumor development and therapy resistance, by preclinical genetics or pharmacologic interventions we hope to uncover new vulnerabilities of these cells, which could be exploited in future clinical development.
Immunometabolic regulation of lung cancer
Lung cancer is the leading cause of cancer deaths worldwide in both women and men. Non-small-cell lung cancer (NSCLC) accounts for 80% of all lung cancer cases, with adenocarcinoma (LUAD) being the major subtype. General failure of response to conventional therapies, a limited number of treatment options, and diagnosis at a late stage are all hallmarks of this devastating disease. Hence, a better understanding of lung cancer is greatly needed. Our research laboratory focuses on the immunologic and metabolic perturbations of lung cancer, with an emphasis on tumor-associated neutrophils (TAN), innate immune cells that may be critical for tumor development and therapy resistance.
Neutrophils in lung cancer: from discovery to functional characterization
Neutrophils are the most abundant immune cells circulating in our body. In contrast to their recognised, crucial contribution in the fight against pathogens, their role in cancer development is less clear. Developing and using multi (16)-color flow cytometry coupled to unbiased analyses, our laboratory identified the presence of a strong neutrophil infiltration in a group of large tumors from the Kras(LSL-G12D)/WT; p53Flox/Flox mouse model of human LUAD.
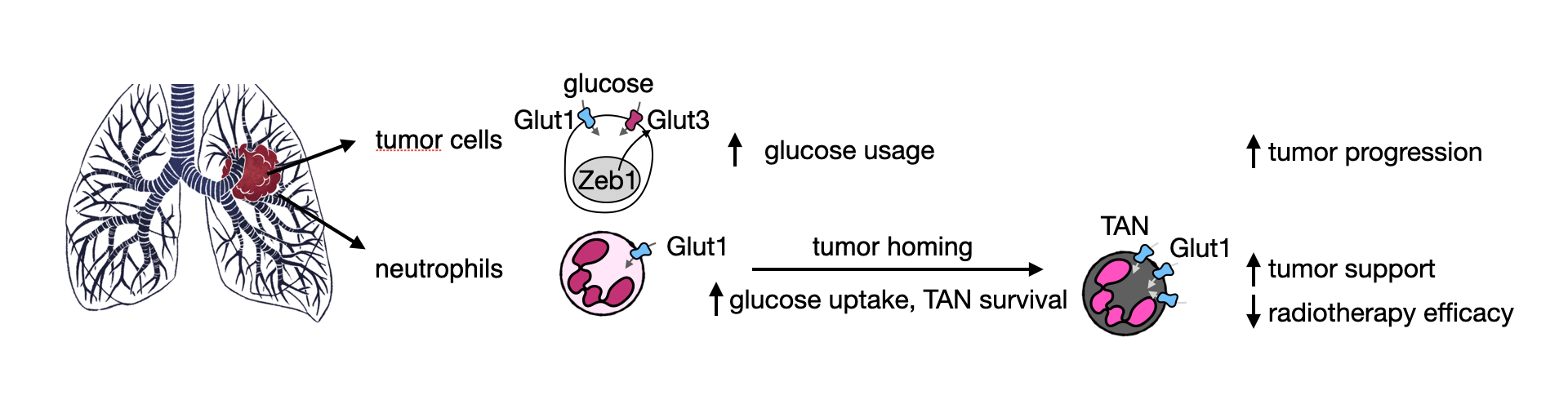
Functionally, we identified that TANs trigger an immune exclusion preventing the success of immunotherapy and communicate with tumor cells to foster tumor growth (Cell Reports 2017). To better study neutrophils in mice, we developed an efficient, specific and long-lasting neutrophil depletion methodology (Nature Communications 2020). To understand if and how TANs change in comparison to neutrophils, a knowledge that may lead to TAN targeting, we took advantage of our studies on tumor metabolism, where we identified the up-regulation of high-affinity glucose transporters in tumor cells from several lung and liver subtypes (Cancer & Metabolism 2014; EMBO Molecular Medicine 2017). By reasoning that TANs may also undergo a metabolic rewiring, we identified an increased glucose uptake and usage by this cell type compared to normal neutrophils. Specifically, loss of the glucose transporter GLUT1 in TANs diminished tumor growth and increased tumor sensitivity to radiotherapy (Cancer Research 2021). Many research questions remain.
-
- How can we identify functionally different TANs?
- What is the cellular language used by TANs to communicate with tumor cells and other cells of the tumor ecosystem?
- How similar and different are TANs when comparing primary tumors from metastases, or lung cancer from other cancers?
- Can we efficiently target pro-tumor TANs without affecting normal neutrophils?
- Which new TAN-targeting compounds could be used in combination with other treatments?
We hope answering these and other research questions will increase our knowledge about the biology of TANs and will help guide new treatment strategies for future clinical use.
